Definition of Software as a Medical Device (SaMD)
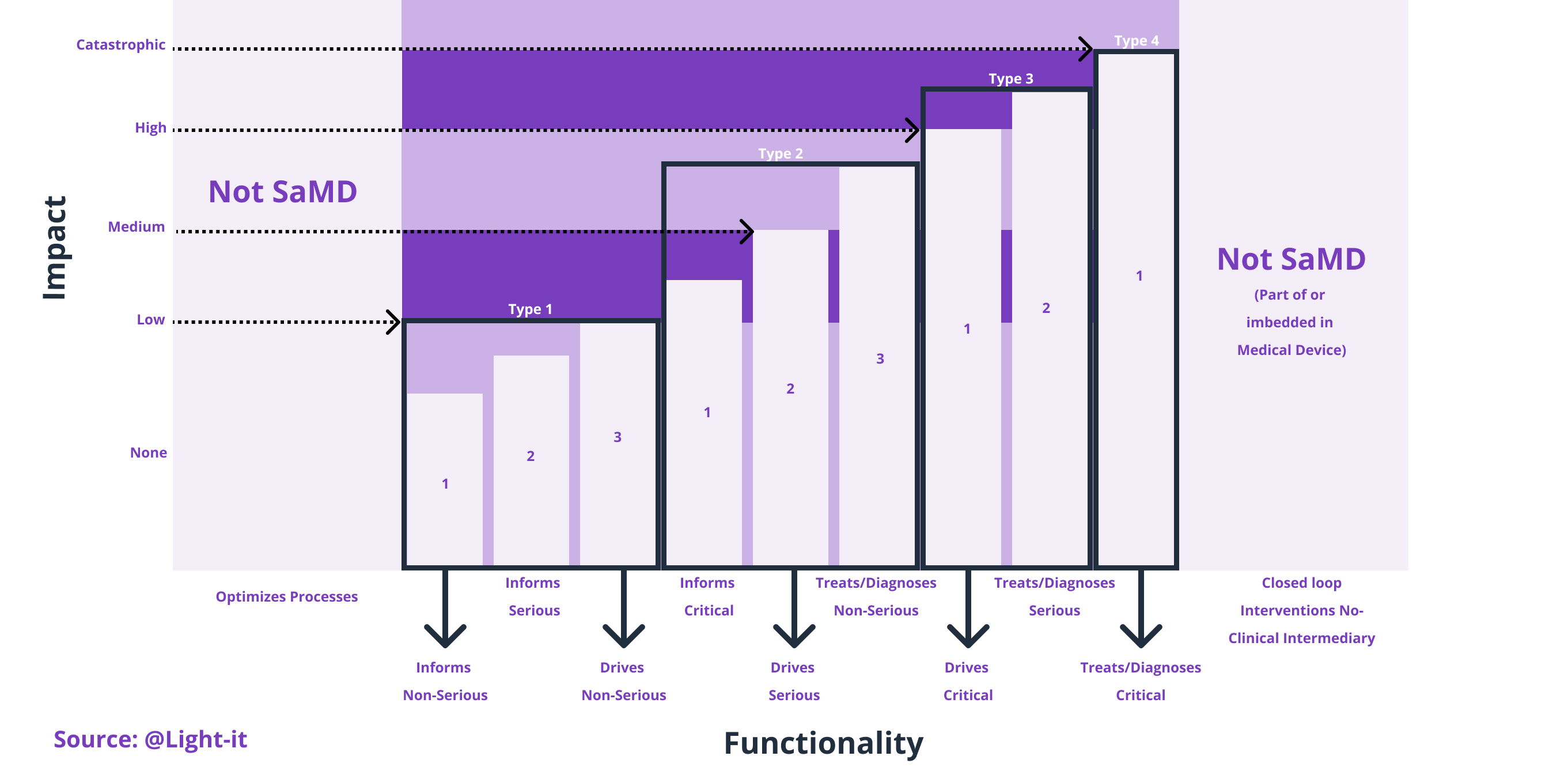
There are four different types of medical device software: Software in a Medical Device (SiMD) which is part of medical hardware or equipment, then there is Software as an Accessory to a Medical Device, in third place Software that is not a Medical Product by itself and lastly, there is Software as a Medical Device (SaMD). This last one is called Standalone Software, and it is software that is used as a medical product without being part of a hardware medical device. Still, it can be used to cooperate with the hardware medical devices and integrate with the medical system.
Specifically, Software as a Medical Device (SaMD) is medical software regulated by the Food and Drug Administration (FDA) in the United States, and it's used to diagnose, prevent, or treat medical conditions. In which computer programs act as medical equipment that can perform general medical functions without being part of a medical team or in a hardware medical device (only on smartphones, computers, tablets, smartwatches, etc.). Healthcare providers can use SaMD to make medical decisions or patients to monitor their health. Indeed, SaMD can manipulate medical information and be part of the treatment itself.
Types and examples of Software as a Medical Device (SaMD)
For example, a mobile app that uses the microphone to analyze your breathing and detect medical conditions or symptoms is considered SaMD.
Other examples of Software as a Medical Device:
- Telemedicine platforms. They allow patients to consult remotely with their physicians by distance via video call or other communication technologies.
- Medical imaging software. They can diagnose medical conditions by analyzing images such as X-rays and MRIs.
- Diagnostic tools. These use the information provided by the patient or collected from medical devices to diagnose medical conditions, such as diabetes or heart disease.
- Disease management tools. They help patients manage chronic conditions, such as asthma or high blood pressure, by providing reminders, tracking symptoms, diving education, and support.
What is not SaMD?
For instance, if the app collects the data with no medical purposes or software used for communications between physicians, hospital or clinic management objectives, such as scheduling, are not considered Software as a Medical Device (SaMD).

Benefits of Software as a Medical Device (SaMD)
- Improved access to medical care: As they can be used without needing specific hardware or devices, SaMD can make medical care more accessible, especially for people who live in remote or underserved areas.
- Better diagnosis and treatment: SaMD can help healthcare providers make more accurate diagnoses and develop more effective treatment plans as they can quickly collect large amounts of data and get instant feedback. It’s important to highlight the fast feedback as it allows interactions that result in different innovation opportunities in the industry. Likewise, when integrated with other medical devices, such as wearables, it can boost the quality of the data collected and make it easier to offer much more efficient treatments.
- Enhanced patient engagement: SaMD can help patients monitor their health, get real-time feedback, and manage their care, leading to better outcomes and improved quality of life.
- Reduced healthcare costs: SaMD can help reduce the cost of medical care by making it more efficient and effective. These tools allow a quicker discovery, management, and automation of specific tasks and, thus, delivery of better treatment to patients while saving time and costs.
- Increased safety and reliability: SaMD is regulated by the FDA, which ensures that it is safe and reliable for use in the medical field
Challenges of Software as a Medical Device (SaMD)
Using software as a medical device (SaMD) has many benefits but also implies several challenges for companies that develop these products and regulatory entities.
As these SaMDs are used for medical purposes that directly impact patients’ health, they must be thoroughly tested and validated before being released to the public to be as safe, private, and effective as possible. There is no doubt that it’s a time-consuming and expensive process but extremely necessary, which puts a barrier to some SaMD companies actually launching their products to market. Following this, once approved, these companies must face extremely strict and specific regulations and requirements to operate correctly, which also implies costs, time, and constant challenges. This can be especially challenging for small or emerging companies needing more resources or expertise to meet these requirements.
Another challenge for SaMD companies is continuously updating and improving their products. As we know, the healthcare industry is experiencing rapid changes, and huge updates are taking place that transform the whole field. This means SaMD products must be regularly updated to reflect the latest scientific knowledge and clinical practices and get the best outcome possible. This can be a significant undertaking, and it can be difficult for SaMD companies to keep up with the rapid pace of change in the medical field.
Moreover, once the SaMD product is launched and updated correctly, the next massive challenge for companies is integrating it safely with the clinic system and regulatory compliance. As with most things in this industry, there is no “one-size-fits-all” approach to managing SaMD products correctly. Thus, the strongest concern of the FDA in this sense is to keep compliance with the stated regulations to ensure privacy and clinical effectiveness without compromising future innovation.
Lastly, as these SaMD are for patients to use as a Standalone Software where they have complete ownership and access to their product, it’s more important than ever that these are as intuitive and easy to use to favor patient’ engagement, experience, and thus, seek for better results.
Regulations of Software as a Medical Device (SaMD)
According to the Food and Drug Administration (FDA, 2022), the Software as a Medical Device WG agreed upon a framework for risk categorization, SaMD Quality Management System, and the clinical evaluation for Software as a Medical Device.
Software as a Medical Device (SaMD) companies
Some well-known companies in this field, according to are:
- MindMaze
- Medtronic
- Viz.AI.
- Siemens Healthcare
- iSchemaView
- Arterys
- Adherium
- Digital Diagnostics
- Allscripts
Best practices of Software as a Medical Device (SaMD)
A number of best practices are recommended for developing and using Software as a Medical Device (SaMD). Some of these include:
- Although it might sound obvious, it’s worth mentioning that all developers must ensure SaMD products are thoroughly tested and validated before they are used in clinical practice. Bad management in this sense might result in a direct health issue for the patient using the product, so prioritize and conduct all clinical trials and testing to ensure that it’s safe and effective for its intended use.
- Following this, although the industry is just taking its first steps in SaMD products and many changes and innovations are yet to come, there is still sufficient data and documents to succeed in creating SaMD products. For example, it’s highly recommended to follow the recognized Standards and Guidance Documents facilitated by Medical Device Regulators Forum (IMDRF), International Organization for Standardization (ISO), and FDA, among others.
- Make sure you keep a high level of rigor when developing these SaMD products, with a deep, end-to-end understanding of the scope and uses of the product. It should be consistent with the level of risk the product implies in the patient’s health and the whole medical system it will integrate. For this, always go back to the regulations, frameworks, and evaluations stated before. Remember that the development process should be strictly monitored yet, sufficiently flexible to adapt to industry changes. A safe way to ensure you keep your development compliant and efficient is to contact an expert development company with deep healthcare knowledge.
- You might be an expert in developing software of different types, but when it comes to a healthcare product, a good practice is to involve a healthcare expert, physician, or professional in it. They will give you a holistic view of all the aspects you take into account while creating the product to make it as smooth and effective as possible (for both the developer and the end-user). Remember that these products are designed to be used by patients or healthcare professionals, so involving them in the process will help you create the most optimum product.
- If your SaMD is targeted at healthcare professionals, a good idea might be to provide ongoing support to help them understand how to make the most out of it and have a smooth experience. Remember that you must validate with your target audience that it's worth developing and adds value to their life. Verify and validate.
- As with all technological products, it’s crucial to keep updated on the latest trends, needs, regulations, and opportunities. Remember, you are not done when the software is validated and released. Specifically, taking advantage of the rapid innovative advances in such specific areas is essential in the medical field. Include them in your product, monitor your performance, and get feedback and insights on improving and testing your product. This will allow you to offer users safe and effective solutions to your target and add as much value as possible.
Future trends of Software as a medical device (SaMD)
Year after year, more healthcare organizations are adopting this software as a medical device (SaMD), and it is still expected to grow in the coming year. Some of the trends that are likely to shape the future of SaMD include the following:
- Increase in personalized medicine. As the healthcare industry moves towards personalized or precision medicine, SaMD products play a significant role in providing tailored, individualized care to patients. The easier and more accessible data becomes, the more precise and sophisticated treatments this software offers, especially when using AI, IoT, and Big Data intelligence. Still, it's essential to consider that this brings up several challenges to unify, regulate and harmonize all the data to make it as safe and positive as possible for the healthcare industry.
- Growth of telemedicine. SaMD products play a crucial role in enabling this trend, providing tools and platforms for healthcare professionals to use and remotely diagnose or treat patients.
- Greater integration of SaMD products with other medical devices. As healthcare organizations move to more connected and interoperable systems, SaMD products will increasingly integrate with other medical devices (wearable sensors, diagnostic equipment, etc.). This will allow the product to show a complete picture of the patient's health and, thus, help professionals make more informed and quicker treatment decisions.
- More AI and machine learning adoption in SaMD products. AI and machine learning will be increasingly common in SaMD products as these technologies enable the development of more advanced, accurate, and sophisticated products based on an individual's unique characteristics.
Overall, the future of SaMD is likely to be marked by continued growth and innovation as healthcare organizations increasingly rely on these products to deliver patients high-quality, effective care.